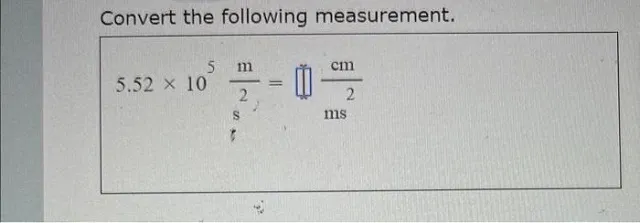
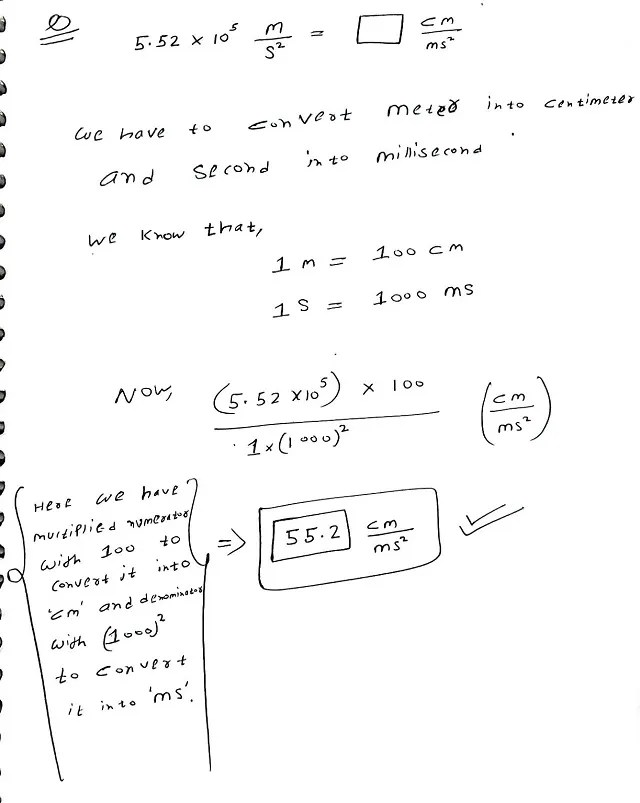
Question
Convert the following measurement
Answer
Measurement Conversions for Chemistry Students: A Comprehensive Guide
Introduction
In the world of chemistry, precise measurements are crucial for accurate experiments and reliable results. Whether you’re working in a lab or studying for exams, mastering measurement conversions is an essential skill for every chemistry student. This comprehensive guide will help you understand the principles behind measurement conversions, provide common conversion factors, and demonstrate practical examples to enhance your proficiency in this fundamental area.
Understanding how to convert between different units of measurement is not just a technical skill but also a fundamental aspect of scientific literacy. Measurements form the backbone of experimental science, and without accurate conversions, data interpretation and comparison can become flawed. This skill is particularly vital in chemistry, where reactions often require precise amounts of substances, and any deviation can lead to significant differences in outcomes.
Furthermore, in today’s globalized world, scientists frequently work with international colleagues and share data across borders. This necessitates a strong command over both the metric and imperial systems, as well as an ability to switch seamlessly between them. For example, a research paper might present results in metric units, while a related industrial application may use imperial units. Thus, competency in measurement conversions is essential for effective communication and collaboration in the scientific community.
Moreover, measurement conversions are foundational for understanding more complex concepts in chemistry. From stoichiometry to thermodynamics, many advanced topics require a solid grasp of basic conversions to apply theoretical knowledge to practical problems. By honing these skills early, students can build a strong foundation that will support their success in more advanced studies and professional applications.
Understanding Measurement Systems
Before diving into conversions, it’s important to understand the measurement systems commonly used in chemistry. The two primary systems are:
- The Metric System: Widely used in science and most countries, the metric system is based on powers of ten, making it straightforward to convert between units. Common units include meters (m) for length, grams (g) for mass, liters (L) for volume, and degrees Celsius (°C) for temperature.
- The Imperial System: Used primarily in the United States, the imperial system includes units such as inches (in), feet (ft), pounds (lbs), and gallons (gal). Converting between imperial and metric units can be challenging but is often necessary in a global scientific context.
Common Conversion Factors
To perform accurate conversions, familiarize yourself with the following common conversion factors:
Length:
- 1 inch = 2.54 centimeters
- 1 foot = 0.3048 meters
- 1 meter = 100 centimeters
Mass:
- 1 ounce = 28.3495 grams
- 1 pound = 0.453592 kilograms
- 1 kilogram = 1000 grams
Volume:
- 1 teaspoon = 4.92892 milliliters
- 1 tablespoon = 14.7868 milliliters
- 1 gallon = 3.78541 liters
Temperature:
- Fahrenheit to Celsius: (°F – 32) × 5/9
- Celsius to Fahrenheit: (°C × 9/5) + 32
- Kelvin to Celsius: K – 273.15
- Celsius to Kelvin: °C + 273.15
Practical Examples
1. Converting Length: Suppose you need to convert 15 inches into centimeters. Using the conversion factor: 15 inches×2.54 cm/inch=38.1 cm15 \text{ inches} \times 2.54 \text{ cm/inch} = 38.1 \text{ cm}15 inches×2.54 cm/inch=38.1 cm
2. Converting Mass: To convert 5 pounds into kilograms: 5 lbs×0.453592 kg/lb=2.26796 kg5 \text{ lbs} \times 0.453592 \text{ kg/lb} = 2.26796 \text{ kg}5 lbs×0.453592 kg/lb=2.26796 kg
3. Converting Volume: Converting 3 gallons into liters: 3 gal×3.78541 L/gal=11.35623 L3 \text{ gal} \times 3.78541 \text{ L/gal} = 11.35623 \text{ L}3 gal×3.78541 L/gal=11.35623 L
4. Converting Temperature: To convert 98°F into Celsius: (98−32)×5/9=36.67°C(98 – 32) \times 5/9 = 36.67°C(98−32)×5/9=36.67°C
Tips for Accurate Conversions
Double-Check Conversion Factors: Always ensure you’re using the correct conversion factor, especially when dealing with less common units.
Use a Calculator: For precise results, use a scientific calculator or conversion tool.
Keep Track of Significant Figures: In chemistry, maintaining the correct number of significant figures is crucial for accuracy.
Practice Regularly: The more you practice, the more comfortable you’ll become with conversions.
Conclusion
Mastering measurement conversions is an indispensable skill for chemistry students. Understanding the differences between the metric and imperial systems, familiarizing yourself with common conversion factors, and practicing with real-world examples will enhance your ability to perform accurate and reliable conversions. This proficiency not only supports your academic success but also prepares you for a career in scientific research, where precision is paramount. Embrace the challenge of mastering measurement conversions, and you’ll find yourself well-equipped to tackle any problem chemistry throws your way.
Keywords for SEO
Measurement conversions, chemistry students, convert measurements, metric system, imperial system, common conversion factors, accurate conversions, chemistry guide, practical examples, significant figures.