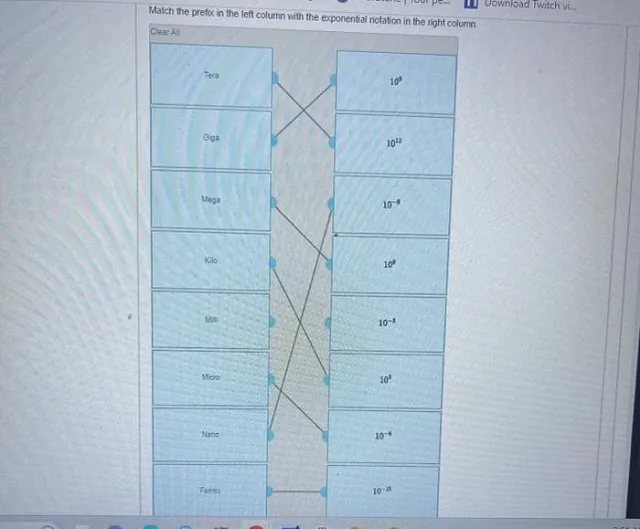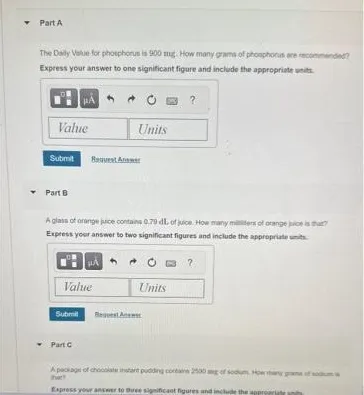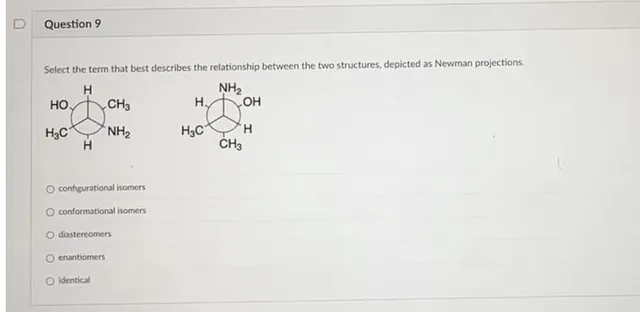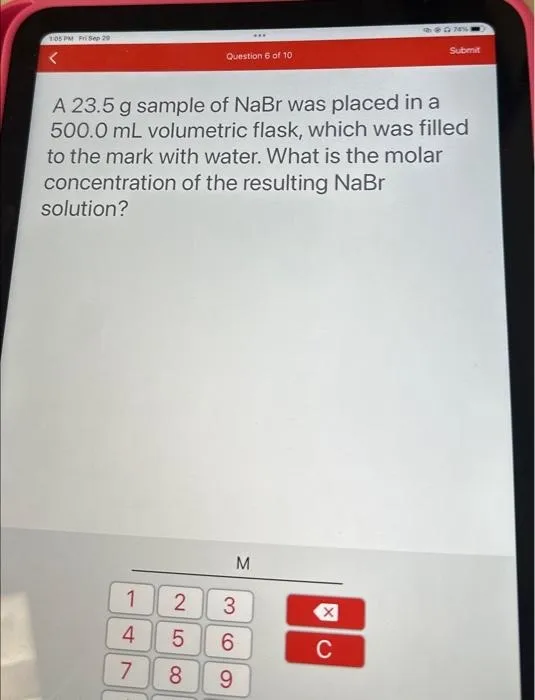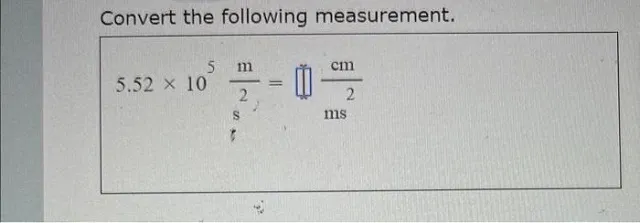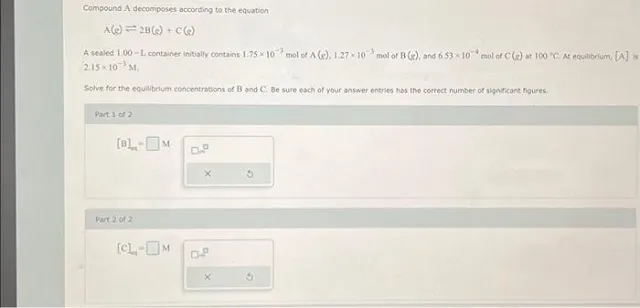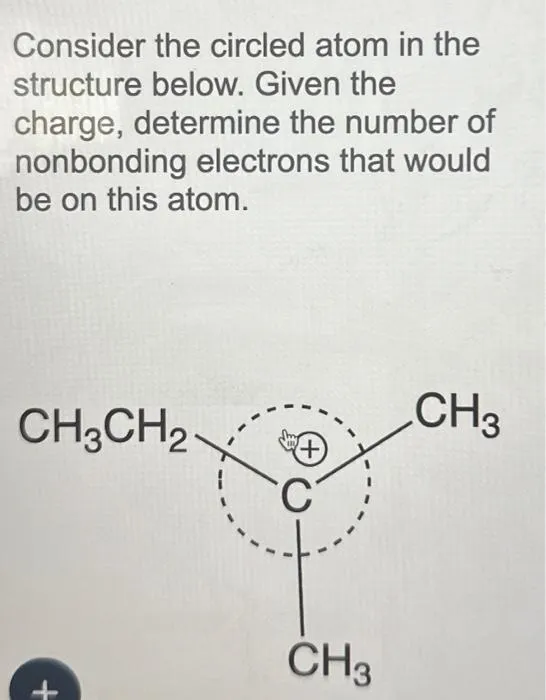
Question Consider the circled atom in the structure below. Given the charge, determine the number of nonbonding electrons that would be on this atom. Answer Determining Nonbonding Electrons for Charged Atoms: A Step-by-Step Guide Understanding the behavior of electrons within molecules is crucial for anyone studying chemistry. One key concept is determining the…