Question
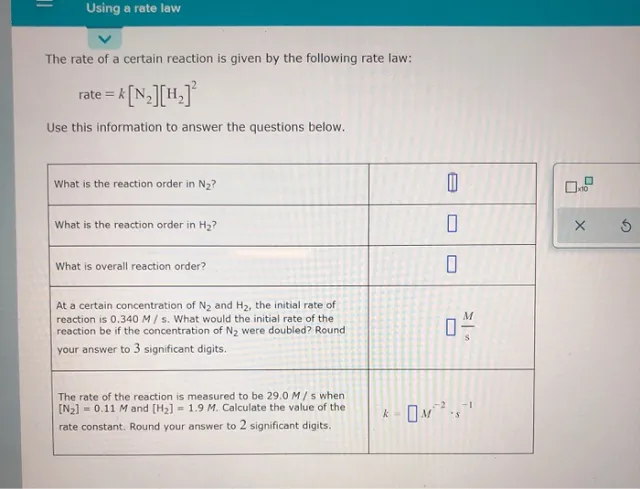
Using a rate law The rate of a certain reaction is given by the following rate law: rate = k[N2][H.] Use this information to answer the questions below. What is the reaction order in N2? 0 What is the reaction order in H2? Х 6 What is overall reaction order? M At a certain concentration of N, and H2, the initial rate of reaction is 0.340 M/s. What would the initial rate of the reaction be if the concentration of N2 were doubled? Round your answer to 3 significant digits. 0% – 1 The rate of the reaction is measured to be 29.0 M/s when [N2] = 0.11 M and [H2] = 1.9 M. Calculate the value of the rate constant. Round your answer to 2 significant digits. k – OM?
Answer

Table of Contents
The Science Behind Rate Law: Exploring Concepts and Formulas
Preface
Understanding the complications of chemical responses is abecedarian to colorful scientific fields, from chemistry to pharmacology. At the heart of these responses lies the conception of rate law, a pivotal aspect that governs the speed and effectiveness of metamorphoses. In this composition, we embark on a trip to unravel the wisdom behind rate law, probing into its core generalities and formulas to give a comprehensive understanding.
What’s Rate Law?
Rate law, also known as rate equation, is an essential principle in chemical kinetics that describes the relationship between the rate of a response and the attention of its reactants. It provides precious perceptivity into how the rate of a response changes with varying attention and helps prognosticate response issues under different conditions.
Key Concepts:
- Rate Determining Step In numerous chemical responses, the overall rate is determined by the slowest step, known as the rate- determining step. relating this step is pivotal for understanding the overall rate expression.
- Order of response The order of a response with respect to a particular reactant is determined by experimental data and represents the exponent of its attention term in the rate equation.
- Rate Constant The rate constant( k) is a proportionality constant that relates the rate of response to the attention of reactants. It’s specific to a particular response at a given temperature.
Formulas
- Rate Law Equation: The general form of a rate law equation is expressed as: rate=𝑘[𝐴]𝑚[𝐵]𝑛rate=k[A]m[B]n Where:
- [𝐴][A] and [𝐵][B] represent the concentrations of reactants A and B, respectively.
- 𝑚m and 𝑛n are the orders of reaction with respect to reactants A and B, respectively.
- 𝑘k is the rate constant.
- Integrated Rate Laws: These equations relate the concentrations of reactants to time and are derived from the rate law equation under different conditions (zero-order, first-order, and second-order reactions).
Applications
- Chemical Kinetics Studies Rate law principles are applied considerably in studying response mechanisms, determining response rates, and expounding response pathways.
- Artificial Processes Understanding rate laws is pivotal for optimizing response conditions in artificial processes to maximize effectiveness and yield.
- Pharmacokinetics In pharmacology, rate law generalities help in understanding medicine immersion, distribution, metabolism, and elimination in the body.
Experimental Determination of Rate Law
Experimental determination of rate law involves conducting controlled experiments to measure the rate of a reaction under different conditions. This process typically entails varying the concentrations of reactants while keeping other factors constant and monitoring the rate of reaction. By analyzing the resulting data, scientists can deduce the order of reaction with respect to each reactant and determine the rate constant. Techniques such as method of initial rates, integrated rate laws, and graphical analysis are employed to obtain accurate rate law expressions, providing valuable insights into the kinetics of chemical reactions.
Conclusion
Rate law serves as a foundation in the study of chemical kinetics, furnishing precious perceptivity into the dynamics of chemical responses. By exploring its generalities and formulas, we gain a deeper understanding of response rates and their dependence on reactant attention. From abecedarian exploration to practical operations in colorful fields, rate law continues to play a vital part in advancing scientific knowledge and technological inventions.