Question
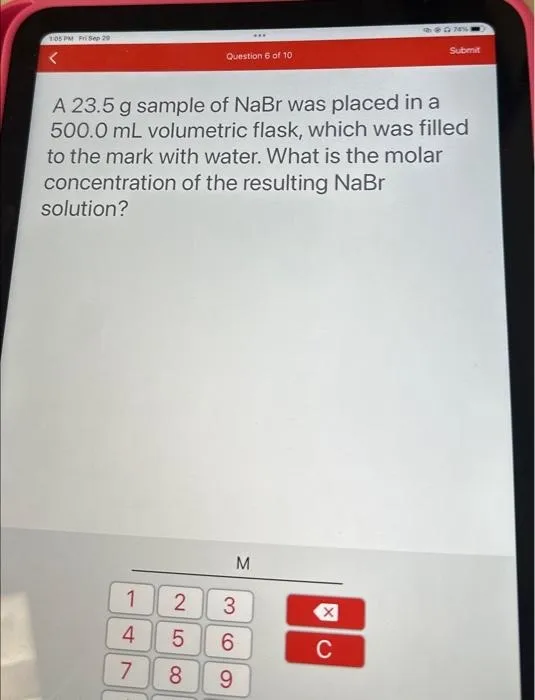
A 23.5 g sample of NaBr was placed in a 500.0 mL volumetric flask, which was filled to the mark with water. What is the molar concentration of the resulting NaBr solution?
Answer
Step 1
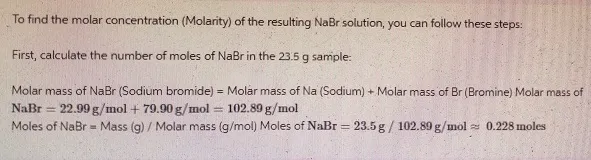
Step 2

Step 3

Answer
The molar concentration of the resulting NaBr solution is approximately 0.456 M.
Molar Concentration Calculation: 23.5 g NaBr in 500 mL Solution
Understanding molar concentration is a fundamental concept in chemistry, particularly when preparing solutions for experiments and reactions. This article provides a step-by-step guide to calculating the molar concentration of a sodium bromide (NaBr) solution prepared by dissolving a 23.5 g sample in a 500.0 mL volumetric flask filled with water.
Introduction
Molar attention, frequently appertained to as molarity, is a measure of the attention of a solute in a result. It’s expressed in intelligencers of solute per liter of result (mol/L). Calculating molarity involves determining the number of moles of the solute and dividing this by the volume of the solution in liters. Molarity is a key concept in many fields, including chemistry, biology, and environmental science, because it allows scientists to precisely describe the concentration of substances in solutions, which is essential for accurate experimental results and reproducible outcomes.
The ability to calculate molar concentration is crucial for anyone working in a laboratory setting. It ensures that solutions are prepared consistently and accurately, which is vital for experimental integrity. Molarity is also important in various industrial processes, such as pharmaceuticals, where the precise concentration of ingredients can significantly impact the efficacy and safety of the final product.
In this example, we will calculate the molarity of a solution made by dissolving 23.5 g of NaBr in 500.0 mL of water. Sodium bromide is a common ionic compound used in various chemical applications, making it a suitable example for this calculation. The principles applied here can be extended to any solute-solvent system, highlighting the versatility and importance of understanding molarity in both academic and practical contexts.
Step-by-Step Calculation
The first step in calculating molarity is to determine the molar mass of the solute, NaBr. The molar mass is the sum of the infinitesimal millions of the rudiments in the emulsion
- Sodium (Na): 22.99 g/mol
- Bromine (Br): 79.90 g/mol
Thus, the molar mass of NaBr is:
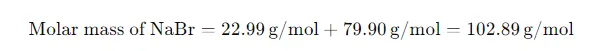
Step 2: Calculate the Moles of NaBr
Next, we need to convert the mass of NaBr (23.5 g) into moles using the molar mass:
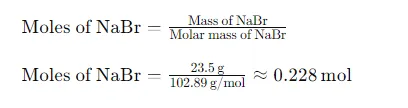
Step 3: Convert the Volume of Solution to Liters
The volume of the solution is given in milliliters (500.0 mL). To use the molarity formula, we need to convert this to liters:
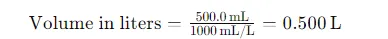
Step 4: Calculate the Molarity of the NaBr Solution
Eventually, we calculate the molarity( M) using the number of intelligencers of NaBr and the volume of the result in liters
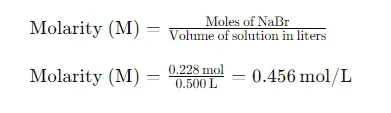
Conclusion
The molar concentration of the NaBr solution is 0.456 mol/L. This calculation involves determining the molar mass of NaBr, converting the mass of NaBr to moles, converting the solution volume to liters, and then applying the molarity formula. Understanding these steps is crucial for accurately preparing chemical solutions and performing various laboratory tasks.
Grasping the concept of molarity not only aids in precise solution preparation but also deepens your understanding of solution chemistry, which is pivotal in various scientific investigations. For instance, in titrations, knowing the exact concentration of solutions allows for accurate determination of unknown concentrations, making molarity a cornerstone of quantitative analysis.
Moreover, molarity plays a significant role in stoichiometry, enabling chemists to predict the outcomes of reactions and determine the required amounts of reactants and products. This knowledge is particularly valuable in industrial settings where efficient and cost-effective production processes are paramount.
In educational settings, mastering molarity calculations enhances students’ problem-solving skills and prepares them for more advanced topics in chemistry and related fields. The ability to perform these calculations with confidence is a fundamental skill that will serve them throughout their academic and professional careers.