Question
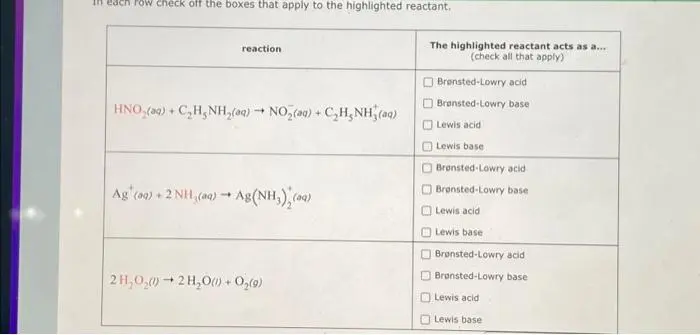
In each row check off the boxes that apply to the highlighted reactant. reaction HNO₂(aq) + C₂H₂NH₂(aq) → NO₂(aq) + C₂H₂NH₂(aq) + Ag* (aq) + 2 NH3(aq) → Ag (NH₂) (aq) 2 H₂O₂(1)→ 2H₂O(1) + O₂(g) The highlighted reactant acts as a… (check all that apply) Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base
Answer
Step 1
We will identify whether the highlighted reactant donates a proton (H⁺) or accepts a proton (H⁺), and whether it donates an electron pair or accepts an electron pair. Based on this information, we can determine the role of the reactant as an acid or a base in the Brønsted-Lowry and Lewis acid-base theories.
Step 2
Reaction: HNO2(aq) + C2H2NH2(aq) ⟶ NO2(aq) + C2H2NH3+(aq)
The highlighted reactant is HNO2(aq).
Explanation:
- It donates a proton (H⁺) in the reaction, acting as a Brønsted-Lowry acid.
- It does not involve the donation or acceptance of an electron pair, so it does not act as a Lewis acid or base
Step 3
Reaction: Ag+ (aq) + 2 NH3(aq) ⟶ Ag (NH3)+ (aq)
The highlighted reactant is NH3(aq).
Explanation:
- It donated a electron (e–) in the reaction, does not acting as a Brønsted-Lowry base.
- It involve the donation of an electron pair, so it act as a Lewis acid or base.
Step 4
Reaction: 2 H2O2(l) ⟶ 2 H2O(l) + O2(g) it is a disproportion type reaction.
The highlighted reactant is H2O2(l).
- It does not donate or accept a proton (H⁺) in the reaction, so it is not a Brønsted-Lowry acid or base and lewis acid or base.
Final Answer
The highlighted reactant is HNO2(aq) – Bronsted-Lowry acid.
The highlighted reactant is NH3(aq)-Lewis base
Table of Contents
Understanding in each row Lewis Acid Lewis Base Interactions in Chemistry
Understanding Lewis Acids and Bases: A Comprehensive Guide” offers an in-depth exploration of the foundational concepts and practical applications of Lewis acid Lewis base theory. From organic synthesis to catalysis, the interactions between electron-deficient Lewis acids and electron-rich Lewis bases play a pivotal role in driving chemical reactions. In each row, check off the boxes to unravel the intricacies of these interactions, as this guide navigates through the principles, reactions, and cutting-edge research surrounding Lewis acids and bases. Whether you’re a seasoned chemist or a curious enthusiast, this comprehensive resource serves as a roadmap for understanding the dynamic world of Lewis acid Lewis base chemistry.
Introduction
Overview of Lewis Acid Lewis Base Theory
The Lewis acid Lewis base theory, introduced by Gilbert N. Lewis in 1923, revolutionized the way chemists understand chemical reactions and interactions. Unlike the traditional Brønsted-Lowry acid-base theory, which focuses on proton donation and acceptance, the Lewis theory broadens the concept by defining acids as electron pair acceptors and bases as electron pair donors. This definition allows for a more comprehensive explanation of various chemical reactions, including those that do not involve protons.
A Lewis acid, by accepting an electron pair, forms a coordinate covalent bond with a Lewis base, which donates the electron pair. This interaction results in the formation of a Lewis adduct. The flexibility of this theory makes it applicable to a wide range of chemical compounds and reactions, from simple ionic interactions to complex organic and inorganic processes. Understanding the nature of Lewis acid Lewis base interactions is crucial for grasping the mechanisms behind catalysis, coordination chemistry, and even biochemical pathways.
In practical applications, the Lewis acid Lewis base theory is invaluable for designing and optimizing industrial processes, such as catalysis in petrochemical refining and polymerization reactions. It also plays a significant role in environmental chemistry, helping to develop methods for pollutant removal and resource recovery. By providing a deeper insight into molecular interactions, the Lewis acid Lewis base theory continues to be a fundamental concept in both theoretical and applied chemistry.
Importance in Chemistry
The Lewis acid Lewis base theory is fundamental to understanding a wide array of chemical reactions and mechanisms. By defining acids as electron pair acceptors and bases as electron pair donors, this theory provides a more versatile framework compared to traditional acid-base theories. This broader perspective is essential for explaining reactions that do not involve protons, thereby enhancing our comprehension of various chemical processes and facilitating the discovery of new reactions and compounds.
In organic chemistry, Lewis acid Lewis base interactions are crucial for many catalytic processes. Lewis acids, such as aluminum chloride and boron trifluoride, are often used as catalysts to accelerate reactions by stabilizing transition states and lowering activation energies. These interactions are pivotal in synthesizing complex organic molecules, making the theory indispensable for pharmaceutical development and material science. Understanding how Lewis acid Lewis base pairs interact allows chemists to design more efficient and selective synthetic routes.
Environmental and industrial chemistry also benefit significantly from the Lewis acid Lewis base theory. It plays a key role in processes such as wastewater treatment, where Lewis acids can help remove contaminants through precipitation or adsorption. Additionally, in industrial applications like polymerization and petrochemical refining, the theory guides the optimization of catalysts to improve yield and efficiency. The ability to manipulate and predict chemical behavior through Lewis acid Lewis base interactions underscores the theory’s critical importance in advancing chemical research and technology.
Definitions and Basic Concepts
What is a Lewis Acid?
In the context of the Lewis acid Lewis base theory, a Lewis acid is defined as a chemical species that can accept an electron pair from a Lewis base to form a coordinate covalent bond. This broader definition encompasses a wide range of compounds, including many that are not considered acids under traditional definitions. Typical examples of Lewis acids include metal cations, such as Fe³⁺ and Al³⁺, and electron-deficient molecules like BF₃ and AlCl₃.
What is a Lewis Base?
In each row the Lewis acid Lewis base theory, a Lewis base is defined as a chemical species that can donate an electron pair to a Lewis acid, resulting in the formation of a coordinate covalent bond. This definition extends beyond the traditional acid-base concept, encompassing a wide variety of electron pair donors. Common examples of Lewis bases include molecules and ions with lone pairs of electrons, such as ammonia (NH₃), water (H₂O), and hydroxide ions (OH⁻).
Comparison with Other Acid-Base Theories
The Lewis acid Lewis base theory offers a broader perspective on chemical interactions compared to traditional acid-base theories like the Arrhenius and Brønsted-Lowry models. While the Arrhenius theory defines acids and bases based on their ability to produce H⁺ and OH⁻ ions in water, the Lewis theory expands this definition by focusing on the transfer of electron pairs. This makes the Lewis acid Lewis base theory applicable to a wider range of reactions, including those that do not involve hydrogen ions.
Characteristics of Lewis Acids and Bases
Common Properties of Lewis Acids
In each row the Lewis acid Lewis base framework, Lewis acids are defined by their ability to accept electron pairs, leading to the formation of coordinate covalent bonds. One common property of Lewis acids is their electron deficiency. This electron deficiency can arise from having an incomplete octet, as seen in compounds like boron trifluoride (BF₃), or from being highly electronegative yet still having available empty orbitals, such as in sulfur dioxide (SO₂). These characteristics make Lewis acids highly reactive towards electron-rich species.
Common Properties of Lewis Bases
In the Lewis acid Lewis base framework, Lewis bases are defined by their ability to donate electron pairs, forming coordinate covalent bonds with Lewis acids. A common property of Lewis bases is the presence of lone pairs of electrons. These lone pairs are typically found in molecules with atoms that have non-bonding electron pairs, such as nitrogen, oxygen, and sulfur. Examples of common Lewis bases include ammonia (NH₃), water (H₂O), and hydroxide ions (OH⁻), all of which readily donate their lone pairs to electron-deficient Lewis acids.
Examples of Lewis Acids and Bases
In each row the Lewis acid Lewis base framework, numerous substances exemplify the roles of electron pair acceptors and donors. Common Lewis acids include boron trifluoride (BF₃), aluminum chloride (AlCl₃), and iron(III) chloride (FeCl₃). These compounds are electron-deficient, enabling them to accept electron pairs from Lewis bases. For instance, BF₃ is frequently used in organic synthesis to facilitate reactions by accepting electron pairs from donor molecules.
Mechanisms of Lewis Acid Lewis Base Interactions
Formation of Lewis Adducts
The formation of Lewis adducts is a fundamental concept in the Lewis acid Lewis base theory. A Lewis adduct results when a Lewis acid and a Lewis base interact, leading to the formation of a coordinate covalent bond. This bond is formed by the donation of an electron pair from the Lewis base to the Lewis acid. For example, when ammonia (NH₃), a Lewis base, donates its lone pair of electrons to boron trifluoride (BF₃), a Lewis acid, they form the Lewis adduct NH₃BF₃.
Electronic and Molecular Structure Changes
In each row the Lewis acid Lewis base theory, the interaction between an acid and a base leads to significant electronic and molecular structure changes. When a Lewis base donates its electron pair to a Lewis acid, it results in the formation of a new coordinate covalent bond. This electron pair donation can cause a redistribution of electron density, altering the electronic structure of both the Lewis acid and the Lewis base. For instance, when ammonia (NH₃) donates an electron pair to boron trifluoride (BF₃), the electron-deficient boron atom becomes more stable, and the electronic environment around both molecules changes.
Energy Considerations in Lewis Interactions
Energy considerations are crucial when examining Lewis acid Lewis base interactions. When a Lewis base donates an electron pair to a Lewis acid, the formation of a coordinate covalent bond releases energy, stabilizing the resulting Lewis adduct. This energy release, known as bond formation energy, is a key factor in the feasibility and spontaneity of these interactions. For instance, the interaction between ammonia (NH₃) and boron trifluoride (BF₃) is energetically favorable due to the significant bond formation energy released when the electron-deficient boron accepts the electron pair from nitrogen.
Examples of Lewis Acid Lewis Base Reactions
Classic Reactions in Organic Chemistry
In organic chemistry, the Lewis acid Lewis base theory plays a fundamental role in explaining and facilitating a variety of classic reactions. One notable example is the Friedel-Crafts alkylation and acylation, where a Lewis acid like aluminum chloride (AlCl₃) acts as a catalyst. In these reactions, AlCl₃ accepts an electron pair from the reactant, forming a complex that makes the electrophile more reactive towards the aromatic ring, leading to the formation of alkylated or acylated products.
Inorganic Chemistry Applications
The Lewis acid Lewis base theory is pivotal in understanding a wide array of reactions and processes in inorganic chemistry. One prominent application is in the formation of coordination compounds, where metal ions (Lewis acids) interact with ligands (Lewis bases). For example, in the complexation of copper(II) ions with ammonia, the Cu²⁺ ion accepts electron pairs from NH₃ molecules, forming a stable complex. This interaction is fundamental in fields like catalysis, material science, and bioinorganic chemistry.
Industrial Processes and Applications
The Lewis acid Lewis base theory finds extensive applications in various industrial processes, where precise control over chemical reactions is paramount. One notable application is in petrochemical refining, where Lewis acids are employed as catalysts in processes like alkylation and polymerization. For instance, aluminum chloride (AlCl₃), acting as a Lewis acid, facilitates the alkylation of aromatic compounds, producing high-octane gasoline additives. These catalytic processes rely on the Lewis acid Lewis base interactions to enhance reaction rates and selectivity.
Significance in Chemical Reactions
Catalysis and Reaction Pathways
Catalysis, driven by Lewis acid Lewis base interactions, is pivotal in expediting chemical reactions and dictating reaction pathways. Lewis acids, acting as electron pair acceptors, form complexes with Lewis bases, facilitating the activation of specific molecular sites within reactants. This activation lowers the energy barrier for reaction initiation, guiding the progression of the reaction towards desired products. Understanding these intricate interactions enables the design of efficient and selective catalytic systems for diverse applications, from industrial processes to pharmaceutical synthesis.
Role in Synthesis and Analytical Chemistry
In synthesis and analytical chemistry, the role of Lewis acid Lewis base interactions is paramount. Lewis acids, by accepting electron pairs from Lewis bases, catalyze a plethora of synthetic reactions, influencing reaction pathways and product yields. This interaction is particularly crucial in organic synthesis, where Lewis acids activate functional groups, enhancing reactivity and facilitating complex molecule assembly. Furthermore, in analytical chemistry, Lewis acid-base reactions are exploited in techniques like complex metric titrations, where metal ions act as Lewis acids, forming complexes with Lewis base ligands. Understanding and harnessing these interactions are instrumental in both synthesis and analysis, shaping advancements in chemical research and technology.
Environmental and Biological Relevance
In environmental and biological contexts, Lewis acid Lewis base interactions play a significant role in various processes and phenomena. In environmental chemistry, Lewis acids, often in the form of metal ions or metal oxides, can act as adsorbents for pollutants, forming stable complexes with Lewis base contaminants. This interaction facilitates pollutant removal from air, water, and soil, contributing to environmental remediation efforts. Moreover, in biological systems, metal ions function as Lewis acids in enzyme catalysis and metalloprotein binding, where they coordinate with Lewis base ligands to facilitate biochemical reactions essential for life. Understanding the environmental and biological relevance of Lewis acid Lewis base interactions is vital for addressing environmental challenges and unraveling the complexities of biological processes.
Advanced Topics
Frontier Molecular Orbital Theory
In chemistry, the Frontier Molecular Orbital (FMO) theory provides insights into the reactivity and stability of molecules, particularly in organic chemistry. This theory explores the interactions between the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) of reactants. In the context of Lewis acid Lewis base interactions, FMO theory helps predict which molecules are likely to act as electron donors (HOMO) and acceptors (LUMO), thus identifying potential Lewis acids and bases in reactions. By understanding these electronic interactions, chemists can design more efficient synthetic routes and predict reaction outcomes with greater precision.
Hard and Soft Acids and Bases (HSAB) Concept
In chemistry, the Hard and Soft Acids and Bases (HSAB) concept provides a framework for understanding the interactions between Lewis acids and bases based on their relative softness or hardness. Hard acids and bases have higher electronegativity and smaller atomic or ionic radii, while soft acids and bases possess lower electronegativity and larger atomic or ionic radii. According to the HSAB principle, hard acids preferentially interact with hard bases, and soft acids with soft bases. This concept helps predict and rationalize the stability and selectivity of Lewis acid Lewis base interactions in various chemical reactions, from coordination chemistry to catalysis, guiding the design of more efficient and selective processes.
Spectroscopic and Computational Analysis
In spectroscopic and computational analysis, the study of Lewis acid Lewis base interactions provides valuable insights into molecular structures, dynamics, and reactivity. Spectroscopic techniques such as infrared (IR), nuclear magnetic resonance (NMR), and X-ray crystallography offer direct observations of Lewis acid-base complexes, revealing information about bond lengths, angles, and intermolecular forces. Computational methods, including density functional theory (DFT) and molecular dynamics (MD) simulations, complement experimental data by providing detailed theoretical models of Lewis acid-base interactions. By combining spectroscopic and computational analyses, researchers can elucidate the mechanisms underlying complex chemical processes, aiding in the design of novel catalysts, materials, and pharmaceuticals with tailored properties and functionalities.
Practical Applications
Lewis Acid Catalysis in Industry
In industrial chemistry, Lewis acid catalysis plays a crucial role in accelerating various chemical reactions. Lewis acids, acting as electron pair acceptors, facilitate reactions by interacting with Lewis bases to form stable complexes. These catalytic processes are widely employed in the production of pharmaceuticals, polymers, and specialty chemicals. By harnessing Lewis acid Lewis base interactions, industrial chemists can design efficient and selective catalytic systems, leading to improved yields and reduced energy consumption in manufacturing processes.
Environmental Chemistry Applications
In environmental chemistry, the principles of Lewis acid Lewis base interactions are utilized in various applications aimed at pollution control and remediation. Metal ions, acting as Lewis acids, can form complexes with Lewis base pollutants in air, water, and soil, facilitating their removal through adsorption or precipitation processes. These interactions are instrumental in wastewater treatment, soil remediation, and air purification efforts. By leveraging Lewis acid Lewis base chemistry, environmental scientists can develop effective strategies for mitigating pollution and safeguarding ecosystems and human health.
Pharmaceutical and Material Science Uses
In pharmaceutical and material science fields, understanding Lewis acid Lewis base interactions is vital for designing innovative drugs and materials. Lewis acids, such as metal ions or metal complexes, can coordinate with Lewis base functional groups in drug molecules or polymers, influencing their properties and reactivity. These interactions play a crucial role in drug synthesis, where catalysts based on Lewis acids facilitate key transformations. Similarly, in material science, Lewis acid Lewis base chemistry governs the fabrication of advanced materials with tailored properties, such as catalytic surfaces and functional polymers. By harnessing these interactions, researchers can develop new pharmaceuticals and materials with enhanced performance and functionality, driving advancements in healthcare and technology.
Challenges and Future Directions
Current Research Trends
Current research trends in chemistry underscore the significance of Lewis acid Lewis base interactions across various disciplines. From organic synthesis to catalysis and beyond, researchers are exploring novel applications and mechanisms involving these interactions. In organic synthesis, the development of new Lewis acid catalysts and their applications in complex molecule synthesis is a burgeoning area of interest. Additionally, in catalysis, researchers are delving deeper into understanding the role of Lewis acids in reaction mechanisms, aiming to design more efficient and sustainable catalytic systems. By staying at the forefront of Lewis acid Lewis base research, scientists are poised to unlock new avenues for innovation and discovery in chemistry.
Technological Advances
Technological advances in various industries owe much to the intricate dynamics of Lewis acid Lewis base interactions. From catalysis to material science, these interactions form the backbone of innovation. In catalysis, Lewis acids accelerate chemical reactions by accepting electron pairs from Lewis bases, leading to the production of essential chemicals and materials. Moreover, in material science, the controlled manipulation of Lewis acid Lewis base interactions enables the creation of advanced materials with tailored properties, revolutionizing fields like electronics and renewable energy. By harnessing the power of Lewis acid Lewis base chemistry, researchers continue to push the boundaries of technology, driving progress and shaping the future.
Future Prospects in Chemistry
The future of chemistry holds promising prospects, with Lewis acid Lewis base interactions poised to play a central role in advancing scientific frontiers. From sustainable catalysis to precision drug design, researchers envision harnessing these interactions to address pressing global challenges. By leveraging the principles of Lewis acid Lewis base chemistry, scientists aim to develop greener synthetic methodologies, create innovative materials with tailored properties, and unlock new avenues for drug discovery and delivery. With a focus on collaboration and interdisciplinary research, the future of chemistry shines brightly with possibilities fueled by the dynamic interplay of Lewis acids and bases.
Conclusion
Recap of Key Points
In summary, the concept of Lewis acid Lewis base interactions forms the cornerstone of modern chemistry, influencing a myriad of reactions and applications. From catalysis to environmental remediation, these interactions drive innovation and progress across diverse fields. Understanding the principles of Lewis acid Lewis base chemistry enables researchers to design more efficient catalysts, develop novel materials, and tackle pressing environmental and healthcare challenges. As we look ahead, the dynamic interplay of Lewis acids and bases promises to continue shaping the landscape of chemistry, opening new avenues for discovery and advancement.
The Continuing Relevance of Lewis Acid Base Theory
The enduring relevance of Lewis acid Lewis base theory underscores its foundational importance in understanding chemical interactions and driving scientific progress. From its inception to the present day, this theory has provided a versatile framework for elucidating reaction mechanisms, designing catalysts, and exploring new frontiers in chemistry. As research advances and new challenges emerge, the principles of Lewis acid Lewis base interactions remain indispensable, guiding the development of innovative technologies and solutions across diverse fields. In an ever-evolving scientific landscape, the timeless insights of Lewis acid Lewis base theory continue to shape the trajectory of chemistry, paving the way for future discoveries and breakthroughs.
References
Suggested Reading and Resources
For those interested in delving deeper into the realm of Lewis acid Lewis base interactions, there are several valuable resources available. Books such as “Lewis Acids in Organic Synthesis” by E. J. Corey and “Lewis Basicity and Affinity Scales: Data and Measurement” by Christian Laurence offer comprehensive insights into the theory and applications of Lewis acids and bases. Additionally, academic journals like the “Journal of the American Chemical Society” and “Chemical Reviews” regularly publish research articles and reviews on topics related to Lewis acid Lewis base chemistry. Online databases like PubMed and Web of Science provide access to a vast array of scientific literature, including recent studies and advancements in the field. By exploring these resources, researchers and enthusiasts can stay abreast of the latest developments and deepen their understanding of Lewis acid Lewis base theory.
Academic Papers and Textbooks
For those seeking comprehensive insights into Lewis acid Lewis base interactions, academic papers and textbooks serve as invaluable resources. Textbooks such as “Introduction to Coordination Chemistry” by Geoffrey A. Lawrance and “Inorganic Chemistry” by Gary L. Miessler and Paul J. Fischer offer thorough explanations of the theory and applications of Lewis acids and bases. Additionally, academic papers published in reputable journals like “Chemical Reviews” and “Journal of the American Chemical Society” provide in-depth analyses of specific aspects of Lewis acid Lewis base chemistry, from reaction mechanisms to catalytic applications. By consulting these scholarly sources, researchers and students can gain a deeper understanding of the fundamental principles and emerging trends in this dynamic field.
Thank You for visit StudyHW