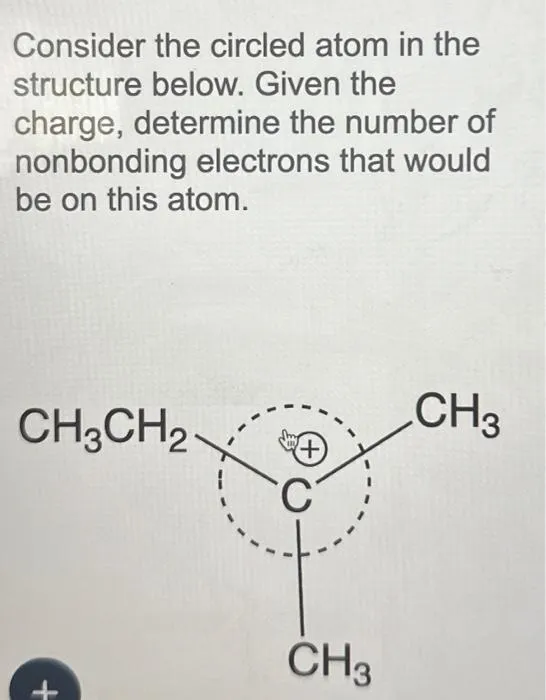
Question
Consider the circled atom in the structure below. Given the charge, determine the number of nonbonding electrons that would be on this atom.
Answer
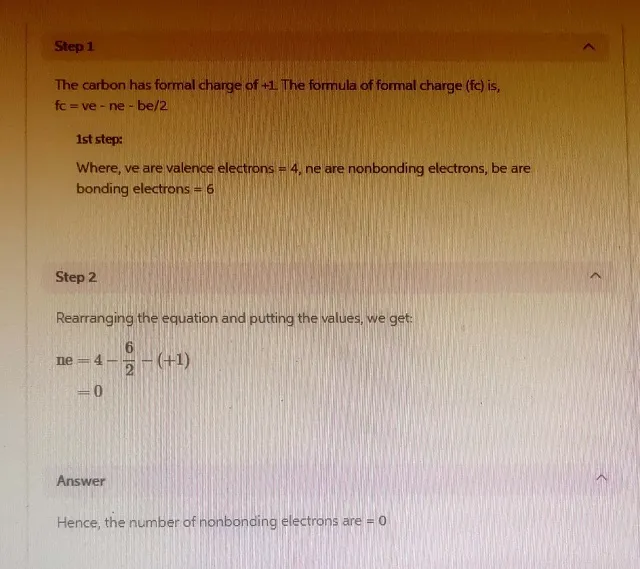
Table of Contents
Determining Nonbonding Electrons for Charged Atoms: A Step-by-Step Guide
Understanding the behavior of electrons within molecules is crucial for anyone studying chemistry. One key concept is determining the number of nonbonding electrons (also known as lone pairs) on an atom, especially when the atom carries a charge. Nonbonding electrons play a vital role in the shape, reactivity, and properties of molecules. This guide will walk you through the steps to determine the number of nonbonding electrons on a charged atom, using fundamental principles of chemistry.
What Are Nonbonding Electrons?
Nonbonding electrons are electrons in an atom that do not participate in bonding with other atoms. They are found in pairs in the outermost electron shell and are responsible for the electron density around an atom. These lone pairs are significant because they influence the molecule’s geometry, polarity, and interactions with other molecules.
Step 1: Determine the Atom’s Valence Electrons
The first step in determining the number of nonbonding electrons is to find the number of valence electrons for the atom in question. Valence electrons are the electrons in the outermost shell of an atom that can participate in bonding. For elements in the main groups of the periodic table, the number of valence electrons corresponds to the group number. For example, oxygen, which is in Group 16, has six valence electrons.
Step 2: Account for the Atom’s Charge
Next, you need to consider the charge on the atom. The charge indicates whether the atom has gained or lost electrons. If the atom has a negative charge (an anion), it has gained electrons, so you should add the number of extra electrons to the total count of valence electrons. If the atom has a positive charge (a cation), it has lost electrons, so you should subtract the number of lost electrons from the total count.
For example, if an oxygen atom has a -1 charge (O⁻), it means the atom has gained one electron, giving it a total of 7 valence electrons instead of 6.
Step 3: Determine the Bonding Electrons
To calculate the number of nonbonding electrons, you must first determine how many electrons are involved in bonding. Typically, each bond (single, double, or triple) formed by the atom will use up two electrons. Count the total number of bonding pairs around the atom.
For instance, if an atom forms two single bonds, it will use four of its valence electrons in bonding.
Step 4: Subtract Bonding Electrons from Total Valence Electrons
Subtract the number of bonding electrons (calculated in Step 3) from the total number of valence electrons (adjusted for any charge, as in Step 2). The result will give you the number of nonbonding electrons.
For example, if an oxygen atom (O⁻) forms two single bonds, it uses four electrons for bonding. Subtracting these from the 7 valence electrons gives 3 electrons remaining, which means there are 1.5 nonbonding pairs (or 3 nonbonding electrons).
Step 5: Double-Check with the Octet Rule
The octet rule is a useful guideline for verifying your results. Most atoms strive to have 8 electrons in their valence shell to achieve stability, similar to the electron configuration of noble gases. After determining the number of bonding and nonbonding electrons, ensure the atom has 8 electrons around it (if it follows the octet rule). If not, recheck your calculations.
For instance, with 7 electrons in the valence shell (including bonding and nonbonding), the oxygen atom would be stable, confirming that the earlier calculation is correct.
Conclusion
Understanding how to determine the number of nonbonding electrons on a charged atom is fundamental to mastering molecular chemistry. By following this step-by-step guide—starting from identifying the atom’s valence electrons, considering the charge, determining bonding electrons, and finally calculating nonbonding electrons—you can accurately analyze the electron distribution in any molecule. This knowledge not only helps in predicting molecular structure but also in understanding reactivity and interactions, which are essential for various applications in chemistry.