Question
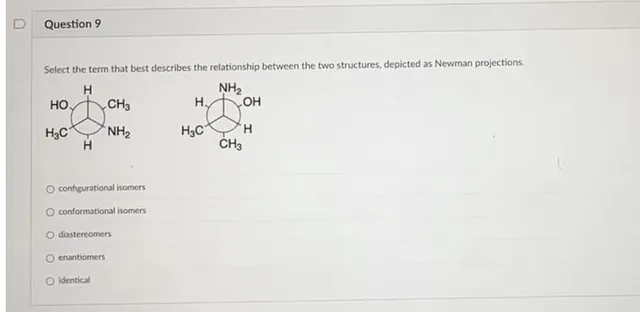
Select the term that best describes the relationship between the two structures, depicted as Newman projections. configurational isomers conformational isomers diastereomers enantiomers Identical
Answer
Step 1
Example of the prediction of relationship between stereo isomers.
Diastereoisomers are non super impossible non mirror images
Enantiomers are non super impossible mirror images
Option C
Explanation:
The given compounds are cis and trans isomers. Since in the first Newmann projection, both -OH and −NH2 are on opposite sides and in the second, they are on same side.
Hence, Cis−Trans isomers are Diastereoisomers
Configurational isomers are R/S and Z/E isomers while conformation isomers are Newmann projections and Chair conformations etc.
Answer
Final answer
Option C
Table of Contents
Understanding Newman Projections: Identifying Isomeric Relationship
In organic chemistry, understanding the spatial arrangement of molecules is essential for predicting their chemical behavior and reactivity. One powerful tool used to visualize these arrangements is the Newman projection. This method allows chemists to see the conformation of a molecule by looking directly down the bond axis. In this article, we will explore how to interpret Newman projections and identify various isomeric relationships, enhancing your ability to analyze molecular structures.
Introduction
Organic molecules are complex three-dimensional structures. Their behavior, reactivity, and properties are heavily influenced by their spatial arrangements. To simplify the visualization of these molecules, chemists use Newman projections. These projections offer a clear perspective on the conformation of molecules by depicting the view along a specific bond. Understanding Newman projections is crucial for identifying isomeric relationships such as configurational isomers, conformational isomers, diastereomers, enantiomers, and identical structures. This article will guide you through the basics of Newman projections and their application in recognizing different types of isomers.
What Are Newman Projections?
Newman projections are diagrams that represent the conformation of a molecule as seen from the perspective of looking down the axis of a specific bond. This visualization technique simplifies the complex three-dimensional structure of a molecule into a more manageable two-dimensional representation.
Components of a Newman Projection
- Front Carbon: Represented by a dot.
- Back Carbon: Represented by a circle.
- Bonds: Lines extending from the dot and circle to depict the bonds to the substituents.
- Substituents: Groups attached to the carbons.
This method is particularly useful for analyzing the rotation around single bonds, which affects the molecule’s overall conformation.
Identifying Isomeric Relationships
Isomers are compounds with the same molecular formula but different arrangements of atoms. The relationship between different isomers can be classified into several categories:
Configurational Isomers
Configurational isomers, or stereoisomers, have the same connectivity of atoms but differ in their spatial arrangement. These isomers cannot be interconverted without breaking and reforming covalent bonds. There are two main types of configurational isomers:
- Enantiomers: Non-superimposable mirror images of each other.
- Diastereomers: Stereoisomers that are not mirror images of each other.
Conformational Isomers
Conformational isomers, or conformers, are different spatial orientations of the same molecule that can be interconverted by rotation around single bonds. These isomers are typically in dynamic equilibrium and do not require breaking covalent bonds.
Identical Molecules
Two structures are considered identical if they can be superimposed on each other without any changes in connectivity or spatial arrangement. Identical molecules have the same chemical and physical properties.
Analyzing Newman Projections
To identify the isomeric relationship between two Newman projections, follow these steps:
- Identify the Front and Back Carbons: Determine which carbons are represented by the dot and the circle.
- Compare the Attached Groups: Examine the groups attached to both the front and back carbons, noting their positions and relative orientations.
- Determine the Isomeric Relationship: Based on the comparison, identify whether the structures are conformational isomers, configurational isomers (enantiomers or diastereomers), or identical.
Example Analysis
Consider two Newman projections of butane:
Structure A:
- Front carbon: methyl group (CH3), hydrogen (H), hydrogen (H)
- Back carbon: methyl group (CH3), hydrogen (H), hydrogen (H)
Structure B:
- Front carbon: methyl group (CH3), hydrogen (H), hydrogen (H)
- Back carbon: methyl group (CH3), hydrogen (H), hydrogen (H)
By rotating the back carbon of Structure A, you can align it with Structure B. Since no bonds need to be broken and the structures are superimposable, these two Newman projections represent identical molecules.
Conclusion
Newman projections are a crucial tool for visualizing molecular conformation and identifying isomeric relationships in organic chemistry. By mastering the interpretation of these projections, chemists can gain deeper insights into molecular geometry and behavior. Whether distinguishing between configurational and conformational isomers or determining if two structures are identical, understanding Newman projections enhances the ability to analyze and predict chemical properties and reactions.