Question
A 23.5 g sample of NaBr was placed in a 500.0 mL volumetric flask. which was filled to the mark with water. What is the molar concentration of the resulting NaBr solufion?
Answer
Step 1
Answer to the given question is as below:
To calculate the molar concentration (Molarity, M) of the resulting NaBr solution, we’ll need to follow these steps:
- Calculate the moles of NaBr in the 23.5 grams sample.
- Determine the volume of the final solution.
- Divide the moles of NaBr by the volume of the solution in liters to find the molar concentration.
Calculate the moles of NaBr in the 23.5 grams sample.
we can use the formula:
Moles(n)=Mass(m) / Molar Mass (M)
The molar mass of NaBr is the sum of the molar masses of sodium (Na) and bromine (Br):
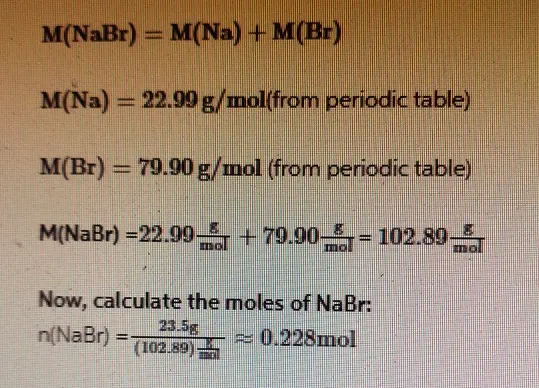
Step 2
Determine the volume of the final solution.
we have placed the 23.5-gram sample in a 500 ml volumetric flask and filled it to the mark with water. The final volume is 500 ml, which is equivalent to 0.5 liters
Explanation:
(since 1 liter = 1000 ml)
Step 3

Answer
Thus, the molar concentration of the resulting NaBr solution is approximately 0.456 M.
Table of Contents
From Mass to Molarity: Calculating Concentration of NaBr Solution
Understanding the molarity of a solution is a fundamental aspect of chemistry, essential for preparing solutions, conducting experiments, and ensuring accurate results. Molarity, defined as the number of moles of solute per liter of solution, is a measure of concentration that helps chemists describe how much of a substance is present in a given volume of liquid. This article will guide you through the step-by-step process of calculating the molarity of a sodium bromide (NaBr) solution, using a 23.5 g sample of NaBr dissolved in a 500.0 mL volumetric flask.
Introduction
In the realm of chemistry, the ability to calculate molarity is a crucial skill. Whether you’re a student, researcher, or professional, understanding how to determine the concentration of a solution is essential for various applications, from titrations to chemical reactions. This article will provide a detailed walkthrough of converting mass to molarity, using a practical example involving sodium bromide (NaBr). We will cover the key concepts, necessary calculations, and provide a comprehensive guide to ensure you can accurately determine molarity in your own experiments.
What is Molarity?
Molarity (M) is a unit of concentration that describes the amount of a solute in a given volume of solution. It is calculated using the formula:

The Task at Hand
Our goal is to calculate the molar concentration of a NaBr solution prepared by dissolving 23.5 grams of NaBr in a 500.0 mL volumetric flask filled to the mark with water. Here’s the step-by-step process:
Step 1: Determine the Molar Mass of NaBr
The molar mass of a compound is the sum of the atomic masses of its constituent elements. Sodium bromide (NaBr) consists of sodium (Na) and bromine (Br).
- Atomic mass of Na = 22.99 g/mol
- Atomic mass of Br = 79.90 g/mol
Molar mass of NaBr = 22.99 g/mol + 79.90 g/mol = 102.89 g/mol
Step 2: Convert the Mass of NaBr to Moles
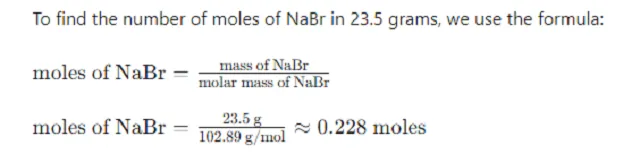
Step 3: Convert the Volume of the Solution to Liters
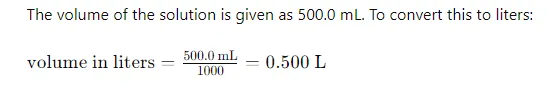
Step 4: Calculate the Molarity

Conclusion
By following these steps, we have determined that the molarity of the NaBr solution is 0.456 M. This calculation demonstrates the importance of understanding the relationship between mass, moles, and volume in determining the concentration of a solution. Accurate calculation of molarity is vital for ensuring the reliability and reproducibility of experimental results in chemistry.
Molarity calculations are foundational skills that support various chemical processes, from preparing solutions for reactions to conducting quantitative analyses. Mastery of these calculations enables chemists to design and perform experiments with precision, ultimately contributing to advancements in research and application.




