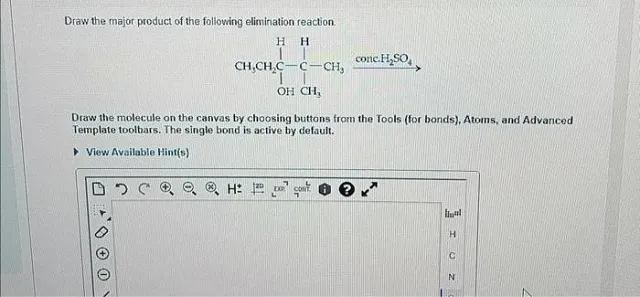
Question
Draw the major product of the following elimination reaction. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.
Answer
Step 1
Organic compounds can be defined as the compounds that contain carbon and hydrogen atoms.
Explanation:
In the given question,, we have been given an alcohol and we have been asked to draw major product of given organic reaction.
Step 2
The major product of the given reaction is shown below.
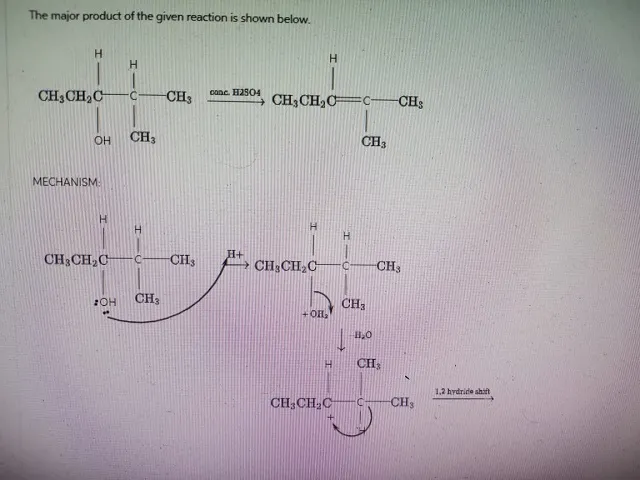
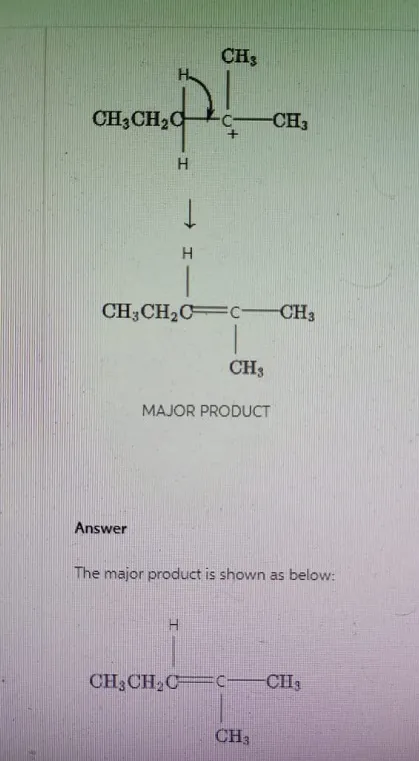
Navigating Elimination Reactions: Bond Strategies and Mechanisms
Introduction
Elimination reactions are fundamental processes in organic chemistry, wherein a molecule loses atoms or groups of atoms to form a new compound. Understanding the intricate mechanisms and strategic bond cleavages involved in these reactions is essential for both academic study and practical applications. In this article, we embark on a journey to navigate the world of elimination reactions, exploring the strategies and mechanisms that govern these transformative processes.
Fundamentals of Elimination Reactions
Elimination reactions typically involve the removal of two substituents from a molecule to form a double bond or π-bond. These reactions are classified into two main types: E1 and E2. E1 reactions proceed via a two-step mechanism involving carbocation intermediates, while E2 reactions occur via a concerted mechanism with simultaneous bond formation and bond breaking.
Bond Strategies in Elimination Reactions
- Choosing the Right Substrate: The nature of the substrate plays a crucial role in determining the feasibility and regioselectivity of elimination reactions. Substrates with bulky or hindered substituents tend to favor E2 mechanisms, whereas substrates with stable carbocations may undergo E1 reactions.
- Positioning of Leaving Groups: The position of leaving groups relative to the site of bond cleavage influences the reaction kinetics and product distribution in elimination reactions. Strategic placement of leaving groups can enhance reaction efficiency and selectivity.
- Effect of Solvent and Temperature: Solvent polarity and temperature exert significant effects on the rate and outcome of elimination reactions. Polar protic solvents favor E1 reactions by stabilizing carbocation intermediates, while polar aprotic solvents promote E2 mechanisms by facilitating nucleophilic attack.
Mechanistic Insights into Elimination Reactions
- E1 Mechanism: In E1 reactions, the rate-determining step involves the formation of a carbocation intermediate through ionization of the substrate. Subsequent deprotonation by a base leads to the elimination of a leaving group and formation of the double bond.
- E2 Mechanism: E2 reactions proceed via a concerted mechanism, wherein the base abstracts a proton from the substrate while simultaneously displacing the leaving group. This concerted bond formation and bond breaking occur in a single step, without the formation of intermediates.
Practical Applications and Importance
Elimination reactions are ubiquitous in organic synthesis, playing a pivotal role in the construction of complex molecules and functional materials. From pharmaceuticals and agrochemicals to polymers and fine chemicals, these reactions find diverse applications across various industries. By harnessing the principles of bond cleavage and mechanism control, chemists can design efficient synthetic routes and optimize reaction conditions for desired outcomes.
Future Perspectives and Research Directions:
As our understanding of elimination reactions continues to evolve, researchers are exploring new frontiers in reaction design and mechanism elucidation. Advances in computational chemistry, spectroscopy, and reaction kinetics are providing unprecedented insights into the intricacies of bond cleavage and molecular rearrangement. Moreover, interdisciplinary collaborations between chemists, engineers, and materials scientists are driving innovation in areas such as catalysis, sustainable synthesis, and drug discovery. By pushing the boundaries of knowledge and technology, scientists are poised to address pressing challenges and unlock new opportunities for innovation in the field of organic chemistry.
Conclusion
Elimination reactions represent a cornerstone of organic chemistry, offering a versatile toolkit for molecular transformation and synthesis. By navigating the strategies and mechanisms underlying these reactions, researchers can unlock new avenues for innovation and discovery in the field of organic synthesis. From fundamental studies of reaction kinetics to practical applications in drug development and materials science, the insights gained from elimination reactions continue to shape our understanding of chemical reactivity and molecular design.